The twin paradox states the following:
There are two identical twins. One of them travels through space in a high-speed rocket. When they return home, the Earth-bound twin has aged more. This is a result of special relativity. Very briefly, this is due to time slowing down as higher speeds are reached, and why Matthew McConaughey returned to Earth only to find his 90-something year old daughter on her dying bed.
This thought experiment has long been exactly that, a though experiment. But recently, we actually were able to learn what happens to twins when one is in space (granted, not in a high-speed rocket, but on the ISS) for almost a year, while the other twin stays on Earth.
Real Space Twinsies
On March 27 2015, astronaut Scott Kelly arrived at the International Space Station (ISS), while his brother, astronaut Mark Kelly, remained on Earth. (One can have a discussion on who was the luckier of the two.) They did the same activities, ate the same things, and followed the same schedule*, the only difference being that Scott was 400 km from the Earth’s surface, travelling at a speed of 7.66 km/s, while Mark was 0 km from the Earth surface, travelling at a speed of merely 460 m/second, as we all are.
340 days later, March 1 2016, Scott returned to Earth. For the full duration of his time on the ISS, as well as after his return, numerous samples were collected and tests were conducted to monitor his health and compare the physiological and biological changes that happened as a consequence of spacelife. Using his twin brother, a perfect genetic duplicate, as a control.

The effects of space
There are many “unusual” aspects about living in space, compared to living on Earth, including the odd noises of the ISS, the isolation (Scott was in contact with a mere 12 people during those 340 days), the ultra-controlled environment, a disruption of the normal body clock (imagine perpetually being jet-lagged because of constant switching of time zones), living in microgravity and the excess of radiation.
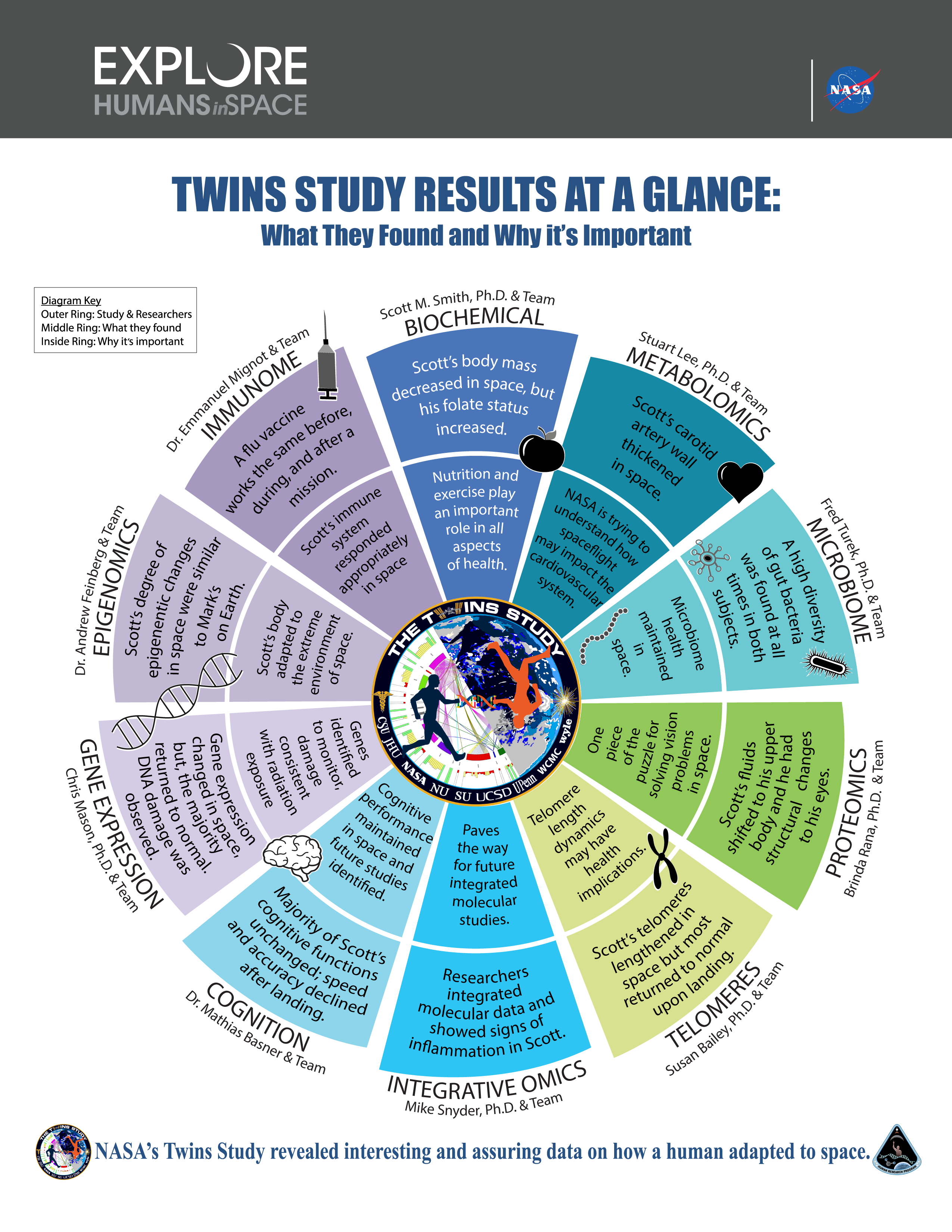
An ultra-combined effort, i.e. a major collaboration between a lot of different labs that looked at all possible aspects of physiological and biological function, the effects of 340 days in space (in this specific set of twins) was published last month. There are a lot of changes that occur to the human body in space, some more severe than others.

There are some changes that don’t really matter much, like changes in the gastrointestinal microbiome and changes in biomass, which were affected during Scott’s time in space, but rapidly returned to normal after he returned. Not much to worry about.
Mid-level risks included known effects of living in microgravity such changes in bone density (you don’t really need to use your skeletal muscles while floating around) and changes in how the heart pumps around blood (you don’t need to fight gravity to pump blood to the head). NASA already knows this and therefore has a rigorous rehabilitation program for returning astronauts to re-acclimatize to Earth’s gravity.
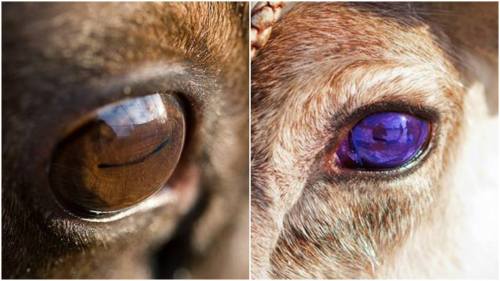
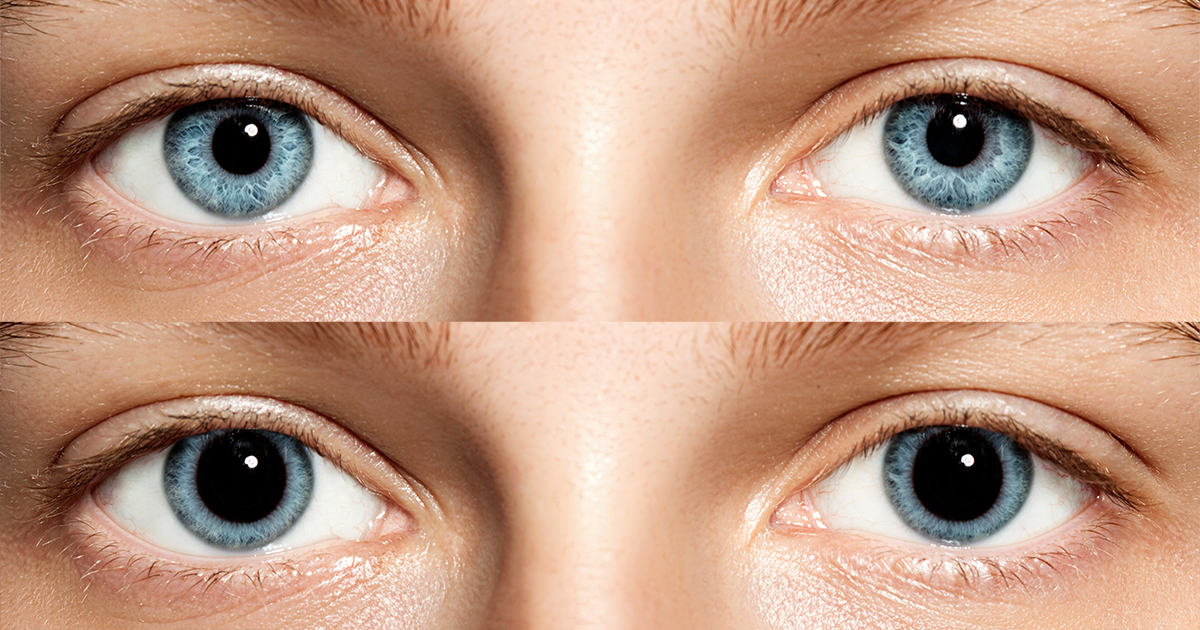
However, it’s the high-risk findings that we all have to worry about, which a mostly due to prolonged floating and prolonged radiation exposure. Due to changes in air pressure as well as that thing I mentioned about blood pumping, a lot of astronauts experience ocular issues after their return, a risk that only increases with increased dwell time off-Earth. This can severely compromise vision. There is also evidence of some cognitive decline. Both those aspects are worrying in the light of long term space travel, we would hope that space-explorers can see and think clearly while carrying out dangerous tasks in dangerous conditions. And that’s without considering a final severe risk…
Who’s the oldest twin?
In addition, the radiation that Scott experienced on ISS is pretty much equivalent to 50 years of normal exposure on Earth. This causes significant genomic instability and DNA damage, and consequentially an increased risk of developing cancer.
One example of this genomic instability has to do with telomeres**. Telomeres are bits of DNA that cap the end of chromosomes. Every time a cell divides, and in the process duplicates its whole DNA library, the telomeres get shorter. When they get too short, the cell can no longer divide. This is something that happens naturally during aging: shortening of telomeres phases out cells until they can no longer divide. Eventually, this leads to cell death.
1 year of space had an odd effect on Scott’s telomeres. Some of them grew longer, while others showed shortening. However, the lengthened telomere returned to normal after Scott’s landing on Earth, while the shortening persisted. So even though Scott was the space twin in our paradox, he seems to have ended up aging faster than Mark…
A lot happens to a body in space
Overall, the results are pretty surprising, prolonged living in space had more of an effect on the human body than researchers expected. And there is probably a lot more to learn, even just with the data collected from Scott and Mark.
On one hand, the twin study showed how resilient and robust the human body is. 91.3% of Scott’s gene expression levels returned to his baseline level within six months of landing, and some of the changes that occurred to his DNA and microbiome were no different than what occurs in high-stress situations on Earth. That’s amazing, the human body has not evolved to survive in space, but it seems to do pretty well considering how outlandish the conditions are!
On the other hand, the prolonged exposure to microgravity and high radiation does have severe effects on human health, leading to increased risk for compromised vision, cardiovascular disease, and cancer development. Even with the rigorous preparation and rehabilitation programs, astronauts go through before and after spaceflight, some of these effects will be impossible to avoid.
The massive study, combining the effort of 84 researchers in 12 different universities is a feat of collaboration (though nothing compared to the black hole telescope, if I’m honest) and it’s definitely a first that the genomes of space vs. Earth could be compared with a true genetic control. This compiled study, and the many pieces of research that are expected to be published in the next year with the results from the individual studies provide crucial insight on the effects of space in the long term. If we think that it takes approximately 1 year for a return journey to Mars, this research is valuable for the health of future astronauts and mankind’s ambition to explore further into space.
Want to know more? Watch NASA’s video on the three key findings, or read more in the Science paper or the NASA website (links below).
Sources:
Markus Löbrich and Penny A. Jeggo. Hazards of human spaceflight. Science 364 (6436) p. 127-128. 2019. DOI: 10.1126/science.aaw7086
Francine E. Garrett-Bakelman, et al. The NASA Twins Study: A multidimensional analysis of a year-long human spaceflight.
Science 364 (6436) eaau8650. 2019. DOI: 10.1126/science.aau8650
Twin study on the NASA website: https://www.nasa.gov/twins-study
Cover image: The International Space Station crosses the terminator above the Gulf of Guinea, image credit NASA
*I remember reading this somewhere, but I cannot find the source anymore. It is thus possible that Mark just went about his normal life. Regardless, it is amazing that NASA had the opportunity to do this experiment with a perfect genetic control.
** Fun fact, my spelling check does not know the word “telomeres” and suggests that I mean “omelettes”. Well, I guess they both get super scrambled up in space? (Eeeeh for an inaccurate joke, sorry).